Q.No:1 TIFR-2013
A certain amount of fluid with heat capacity \(C_F\) \({Joules/^{\circ}C}\) is initially at a temperature \(0^{\circ}C\). It is then brought into contact with a heat bath at a temperature of \(100^{\circ}C\), and the system is allowed to come into equilibrium. In this process, the entropy (in \({Joules/^{\circ}C}\)) of the Universe changes by
(a)
\(100 C_F\)
(b)
\(0\)
(c)
\(0.055 C_F\)
(d)
\(0.044 C_F\)
Check Answer
Option d
Q.No:2 TIFR-2013
A monatomic gas is described by the equation of state
\[
p(V-bn)=nRT
\]
where \(b\) and \(R\) are constants and other quantities have their usual meanings. The maximum density (in moles per unit volume) to which this gas can be compressed is
(a)
\(1/bn\)
(b)
\(b\)
(c)
\(1/b\)
(d)
infinity
Check Answer
Option c
Q.No:3 TIFR-2013
A classical ideal gas, consisting of \(N\) particles (\(N\to \infty\)) is confined in a box of volume \(V\) at temperature \(T\) and pressure \(p\). The probability that, at any instant of time, a small sub-volume \(v_0\) becomes totally void (i.e. no particles inside), due to a spontaneous statistical fluctuation, is
(a)
\(\exp{\left(-v_0/V\right)}\)
(b)
\(\exp{\left(-Nv_0/V\right)}\)
(c)
\(\frac{v_0}{V}\exp{\left(-pV/NT\right)}\)
(d)
\(pv_0/NT\)
Check Answer
Option b
Q.No:4 TIFR-2013
The \(pV\)-diagram for a Carnot cycle executed by an ideal gas with \(C_P/C_V=\gamma>1\) is shown below. Note that \(1, 2, 3\) and \(4\) label the change-over points in the cycle.
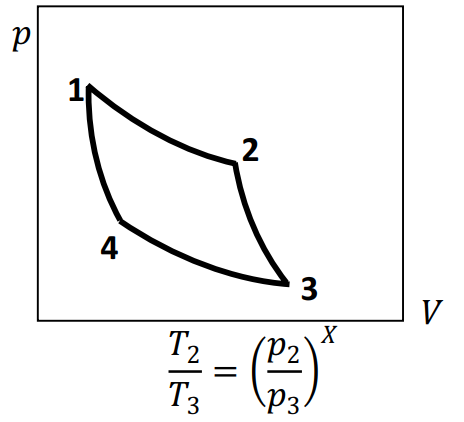
If, for this cycle, then \(X=\)
(a)
\(1-1/\gamma\)
(b)
\(0\)
(c)
\(1\)
(d)
\(-1/\gamma\)
Check Answer
Option a
Q.No:5 TIFR-2014
One mole of an ideal gas undergoes the cycle \(ACBA\) shown in the \(pV\) diagram below.
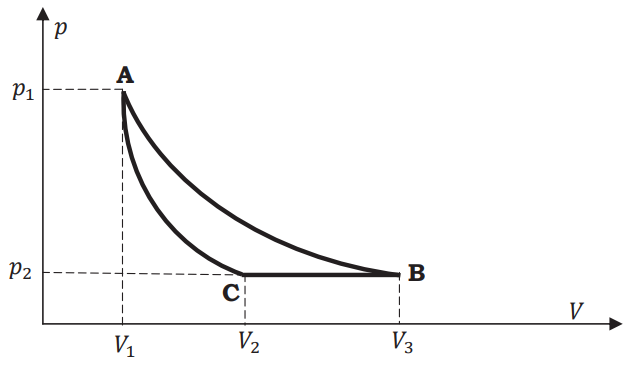
One of the curved lines in the cycle represents an isothermal change at temperature \(T\), while the other represents an adiabatic change. The net heat gained by the gas in this cycle is
(a)
\(-p_2(V_3-V_2)+RT\ln{\frac{V_2}{V_1}}\)
(b)
\(-p_2(V_3-V_2)+RT\ln{\frac{V_3}{V_1}}\)
(c)
\(-p_2(V_3-V_2)+\gamma RT(V_2^{1-\gamma}-V_1^{1-\gamma})\)
(d)
\((p_1 V_1-p_2 V_2)-RT\ln{\frac{V_3}{V_1}}\)
Check Answer
Option b
Q.No:6 TIFR-2014
An ideal gas at a temperature \(T\) is enclosed in a rigid container whose walls are initially at temperature \(T_1\), where \(T_1<T\). The walls are covered on the outside with perfect thermal insulation and the system is allowed to come to equilibrium. The pressure exerted by the gas on the walls of the container
(a)
remains constant throughout.
(b)
is lower at the initial stage than at the final stage.
(c)
is higher at the initial stage than at the final stage.
(d)
is the same at the initial and final stages.
Check Answer
Option c
Q.No:7 TIFR-2014
A thermally-insulated container of volume \(V_0\) is divided into two equal halves by a non-permeable partition. A real gas with equation of state
\[
b^3\left(p+\frac{a^2}{V^3}\right)=nRT
\]
where \(a\) and \(b\) are constants, is confined to one of these halves at a temperature \(T_0\). The partition is now removed suddenly and the gas is allowed to expand to fill the entire container. The final temperature of the gas, in terms of its specific heat \(C_V\), will be
(a)
\(T_0-\frac{3a^2}{2C_V V_0^2}\)
(b)
\(T_0-\frac{2a^2}{3C_V V_0^2}\)
(c)
\(T_0+\frac{3a^2}{2C_V V_0^2}\)
(d)
\(T_0+\frac{2a^2}{3C_V V_0^2}\)
Check Answer
Option a
Q.No:8 TIFR-2015
The equation of state of a gas is given by
\[
V=\frac{RT}{P}-\frac{b}{T}
\]
where \(R\) is the gas constant and \(b\) is another constant parameter. The specific heat at constant pressure \(C_P\) and the specific heat at constant volume \(C_V\) for this gas is related by \(C_P-C_V=\)
(a)
\(R\)
(b)
\(R\left(1+\frac{RT^2}{bP}\right)^2\)
(c)
\(R\left(1+\frac{bP}{RT^2}\right)^2\)
(d)
\(R\left(1-\frac{bP}{RT^2}\right)^2\)
Check Answer
Option c
Q.No:9 TIFR-2015
An ideal diatomic gas is initially at a temperature \(T=0^{\circ}C\). Then it expands reversibly and adiabatically to \(5\) times its volume. Its final temperature will be approximately
(a)
\(-180^{\circ}C\)
(b)
\(-150^{\circ}C\)
(c)
\(-130^{\circ}C\)
(d)
\(0^{\circ}C\)
Check Answer
Option c
Q.No:10 TIFR-2016
In the temperature range \(100\)--\(1000 C\), the molar specific heat of a metal varies with temperature \(T\) (measured in degree Celsius) according to the formula \(C_p=(1+T/5)\) J-deg \(C^{-1} mol^{-1}\). If \(0.2 kg\) of the metal at \(600 C\) is brought in thermal contact with \(0.1 kg\) of the same metal at \(300 C\), the final equilibrium temperature, in deg C, will be
(a)
\(466\)
(b)
\(567\)
(c)
\(383\)
(d)
\(519\)
Check Answer
Option d
Q.No:11 TIFR-2016
The equation of state for a gas is given by
\[
\left[p+\left(\frac{\alpha N}{V}\right)^2\right](V-\beta N)=Nk_B T
\]
where \(P, V, T, N\), and \(k_B\) represent pressure, volume, temperature, number of atoms and the Boltzmann constant, respectively, while \(\alpha\) and \(\beta\) are constants specific to the gas.
If the critical point \(C\) corresponds to a point of inflexion of the \(p\)-\(V\) curve, then the critical volume \(V_C\) and critical pressure \(p_C\) for this gas are given by
(a)
\(V_C=3\beta N, p_C=\alpha^2/3\beta^2\)
(b)
\(V_C=3\beta N, p_C=\alpha/27\beta^2\)
(c)
\(V_C=3\beta N, p_C=8\alpha^2/27\beta\)
(d)
\(V_C=3\beta N, p_C=\alpha^2/27\beta^2\)
Check Answer
Option d
Q.No:12 TIFR-2016
Two containers are maintained at the same temperature and are filled with ideal gases whose molecules have mass \(m_1\) and \(m_2\) respectively. The mean speed of molecules of the second gas is \(10\) times the r.m.s. speed of the molecules of the first gas. Find the ratio of \(m_1/m_2\), to the nearest integer.
Check Answer
Ans \(118\)
Q.No:13 TIFR-2017
A thermally isolated container stores \(N_2\) gas at \(27.24^{\circ}C\) at one atmospheric pressure. Suddenly the pressure of the gas is increased to two atmospheric pressures. Assuming \(N_2\)to behave as an ideal gas, estimate the change in temperature of the gas, in Celsius degrees (\({}^{\circ}C\)).
Check Answer
Ans \(66\)
Q.No:14 TIFR-2017
One mole of monoatomic ideal gas is initially at pressure \(P_0\) and volume \(V_0\).
The gas then undergoes a three-stage cycle consisting of the following processes:
(i) An isothermal expansion till it reaches volume \(2V_0\), and heat \(Q\) flows into the gas
(ii) An isobaric compression back to the original volume \(V_0\)
(iii) An isochoric increase in pressure till the original pressure \(P_0\) is regained.
The efficiency of this cycle can be expressed as

(a) \(\epsilon=\frac{4Q+2RT_0}{4Q+RT_0}\)
(b)
\(\epsilon=\frac{4Q+2RT_0}{4Q-3RT_0}\)
(c)
\(\epsilon=\frac{4Q-2RT_0}{4Q+RT_0}\)
(d)
\(\epsilon=\frac{4Q-2RT_0}{4Q+3RT_0}\)
Check Answer
Option d
Q.No:15 TIFR-2018
A heat engine is operated between two bodies that are kept at constant pressure. The constant-pressure heat capacity \(C_p\) of the reservoirs is independent of temperature. Initially the reservoirs are at temperature \(300 \hspace{1mm} \text{K}\) and \(402 \hspace{1mm} \text{K}\). If, after some time, they come to a common final temperature \(T_f\), the process remaining adiabatic, what is the value of \(T_f\) (in kelvin)?
Check Answer
Ans \(347\)
Q.No:16 TIFR-2018
A many-body system undergoes a phase transition between two phases \(A\) and \(B\) at a temperature \(T_c\). The temperature-dependent specific heat at constant volume \(C_V\) of the two phases are given by \(C_V^{(A)}=aT^3+bT\) and \(C_V^{(B)}=cT^3\). Assuming negligible volume change of the system, and no latent heat generated in the phase transition, \(T_c\) is
(a)
\(\sqrt{\frac{2b}{c}}\)
(b)
\(\sqrt{\frac{b}{c-a}}\)
(c)
\(\sqrt{\frac{3b}{c-a}}\)
(d)
\(\sqrt{\frac{4b}{c-a}}\)
Check Answer
Option c
Q.No:17 TIFR-2019
A thermally-insulated coffee mug contains \(500 \hspace{1mm}\text{g}\) of warm coffee at \(80^{\circ}C\). Assuming that the heat capacity of this liquid is \(1 \hspace{1mm}\text{cal}\hspace{1mm} \text{g}^{-1}\hspace{1mm} {}^{\circ}\text{C}^{-1}\) and the latent heat of fusion for ice is \(80 \hspace{1mm}\text{cal}\hspace{1mm}\text{g}^{-1}\), the amount of ice that must be dropped into the cup to convert it into cold coffee at \(5^{\circ}\text{C}\) is about
(a)
\(421 \hspace{1mm}\text{g}\)
(b)
\(441 \hspace{1mm}\text{g}\)
(c)
\(469 \hspace{1mm}\text{g}\)
(d)
\(471 \hspace{1mm}\text{g}\)
Check Answer
Option b
Q.No:18 TIFR-2019
An ideal gas engine is run according to the cycle shown in the \(s\)-\(T\) diagram below, where the process from D to A is known to be isochoric (i.e. maintaining \(V=\) constant).

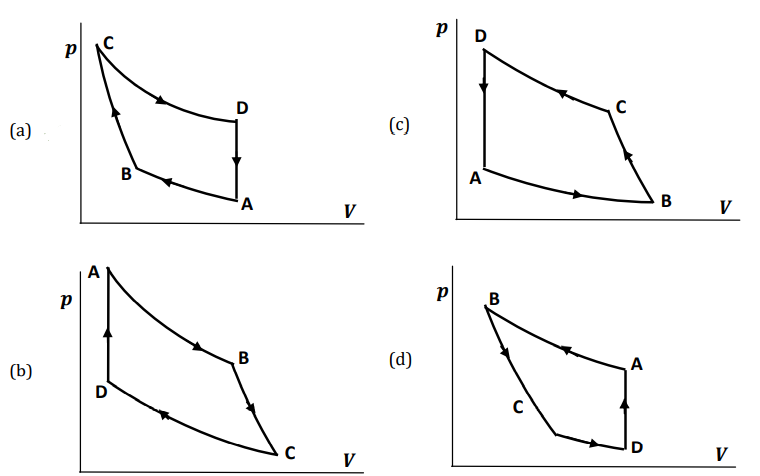
Check Answer
Option a
Q.No:19 TIFR-2020
A gas has the following equation of state
\[
U=\frac{aS^5}{N^2 V^2}
\]
where \(U\) is the internal energy, \(V\) is the volume and \(N\) is the number of particles. Here \(a\) is a constant of the appropriate dimension. It follows that the equation of state of this gas relating its pressure \(P\) to its temperature \(T\) and its density \(\rho=N/V\) is given by
(a)
\(\frac{P^4}{T^5 \rho^2}=\text{constant}\)
(b)
\(\frac{P^5}{T^4 \rho^3}=\text{constant}\)
(c)
\(\frac{P}{T \rho}=\text{constant}\)
(d)
\(\frac{P^3}{T^2 \rho^3}=\text{constant}\)
Check Answer
Option a
Q.No:20 TIFR-2020
An ideal gas is passed through a cyclic process where the corresponding changes in the thermodynamic potentials are plotted on the adjoining graph. Here \(U\) is the internal energy and \(F\) is the Helmholtz free energy.
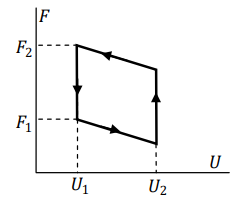
The efficiency of this cycle is given by
(a)
\(1-\frac{U_1}{U_2}\)
(b)
\(1-\exp{\left(-\frac{F_2}{F_1}\right)}\)
(c)
\(1-\frac{U_1}{U_2}\exp{\left(-\frac{F_2}{F_1}\right)}\)
(d)
\(\exp{\left(\frac{U_1}{U_2}\right)}-\exp{\left(-\frac{F_2}{F_1}\right)}\)
Check Answer
Option a
Q.No:21 TIFR-2020
The mean free path \(\lambda\) of molecules of a gas at room temperatures is given approximately by
\[
\lambda=\frac{1}{n\sigma}
\]
where \(n\) is the number density of the molecules and \(\sigma\) is the collision cross-section of two molecules. It follows that the mean free path of air molecules at normal temperature and pressure is of order
(a)
\(500 \hspace{1mm}\mu\text{m}\)
(b)
\(50 \hspace{1mm}\text{nm}\)
(c)
\(0.5 \hspace{1mm}\text{nm}\)
(d)
\(500 \hspace{1mm}\text{fm}\)
Check Answer
Option b
Q.No:22 TIFR-2020
The volume \(V\) of a rectangular box is divided into two equal parts by a solid non-permeable partition \(P\). On one side of the partition \(P\) there is a vacuum, while the other side is filled with a real gas having equation of state
\[
pVe^{a/RTV}=nRT
\]
where \(a\) and \(b\) are constants. The gas was initially at a uniform temperature \(T_0\). Then the partition \(P\) was removed instantaneously, and the gas was allowed to expand to fill the full volume of the box and come to equilibrium.

The final temperature of the gas, in terms of its specific heat \(C_V\) will be
(a)
\(T-\left(\frac{na}{C_V}\right)\ln{2}\)
(b)
\(T+\left(\frac{na}{C_V}\right)\ln{2}\)
(c)
\(T-2n\left(\frac{RTa}{C_V}\right)^{3/2}\)
(d)
\(T+2n\left(\frac{RTa}{C_V}\right)^{3/2}\)
Check Answer
Option a
Q.No:23 TIFR-2021
A boiler of volume \(1.7 \hspace{1mm}\text{m}^3\), when filled with \(1.0 \hspace{1mm}\text{kg}\) of steam at \(100^{\circ}\text{C}\), has a pressure of \(1.0 \hspace{1mm}\text{atm}\). What will be the boiling point of water in this boiler when the pressure is \(2.0 \hspace{1mm}\text{atm}\)?
[The latent heat of vaporization of water is \(2250\times 10^3 \hspace{1mm}\text{J}/\text{kg}\); \(1\hspace{1mm}\text{atm}=10^5 \hspace{1mm}\text{N}/\text{m}^2\)]
(a)
\(128^{\circ}\text{C}\)
(b)
\(118^{\circ}\text{C}\)
(c)
\(78^{\circ}\text{C}\)
(d)
\(88^{\circ}\text{C}\)
Check Answer
Option a
Q.No:24 TIFR-2021
A spherical balloon of radius \(R\) is made of a material with surface tension \(\gamma\) and filled with \(N\) particles of an ideal gas. If the outside air pressure is \(P\), the pressure \(P_b\) inside the balloon is given by
(a)
\(P_b=P+2\gamma/R\)
(b)
\(P_b=P\)
(c)
\(P_b=P-2\gamma/R\)
(d)
\(P_b=P+3\gamma/R\)
Check Answer
Option a
Q.No:25 TIFR-2022
A bicycle tyre is pumped with air to an internal pressure of 6 atm at \(20^\circ C\), at which point it suddenly bursts. Assuming the external pressure to be 1 atmosphere and the subsequent sudden expansion to be adiabatic, the temperature immediately after the burst is approximately
(a)
\(-97.5 ^\circ C\)
(b)
\(-108.5 ^\circ C\)
(c)
\(45.5 ^\circ C\)
(d)
\(216.05 ^\circ C\)
Check Answer
Option a
Q.No:26 TIFR-2023
Consider a sealed but thermally conducting container of total volume \(V\), which is in equilibrium with a thermal bath at temperature \(T\). The container is divided into two equal chambers by a thin partition, which is thermally conducting but impermeable to particles. One of the chambers contains an ideal gas, while the other is a vacuum.
If the partition is removed suddenly and the ideal gas is allowed to expand and fill the entire container, then, once equilibrium has been reached, the entropy per molecule will increase by an amount
(a)
\(+k_B \hspace{0.5mm} ln \hspace{0.5mm} 2\)
(b)
\(\frac{1}{2}k_B \hspace{0.5mm} ln \hspace{0.5mm} 2\)
(c)
\(2k_B \)
(d)
\(-k_B \hspace{0.5mm} ln \hspace{0.5mm} 2\)
